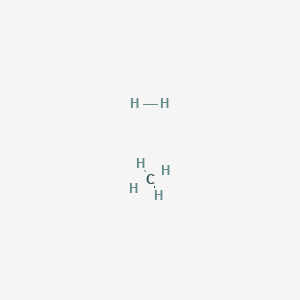
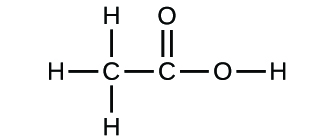
The empirical formula of an organic compound containing carbon and hydrogen is CH2 . The mass of one litre of this organic gas is exactly equal to that of one litre of

SOLVED: A compound contains, by mass, 48.64% carbon, 8.16% hydrogen and 43.209 oxygen The empirical formula of this compound is: CzHsOz CaHgO3 CzH;O CzH;O
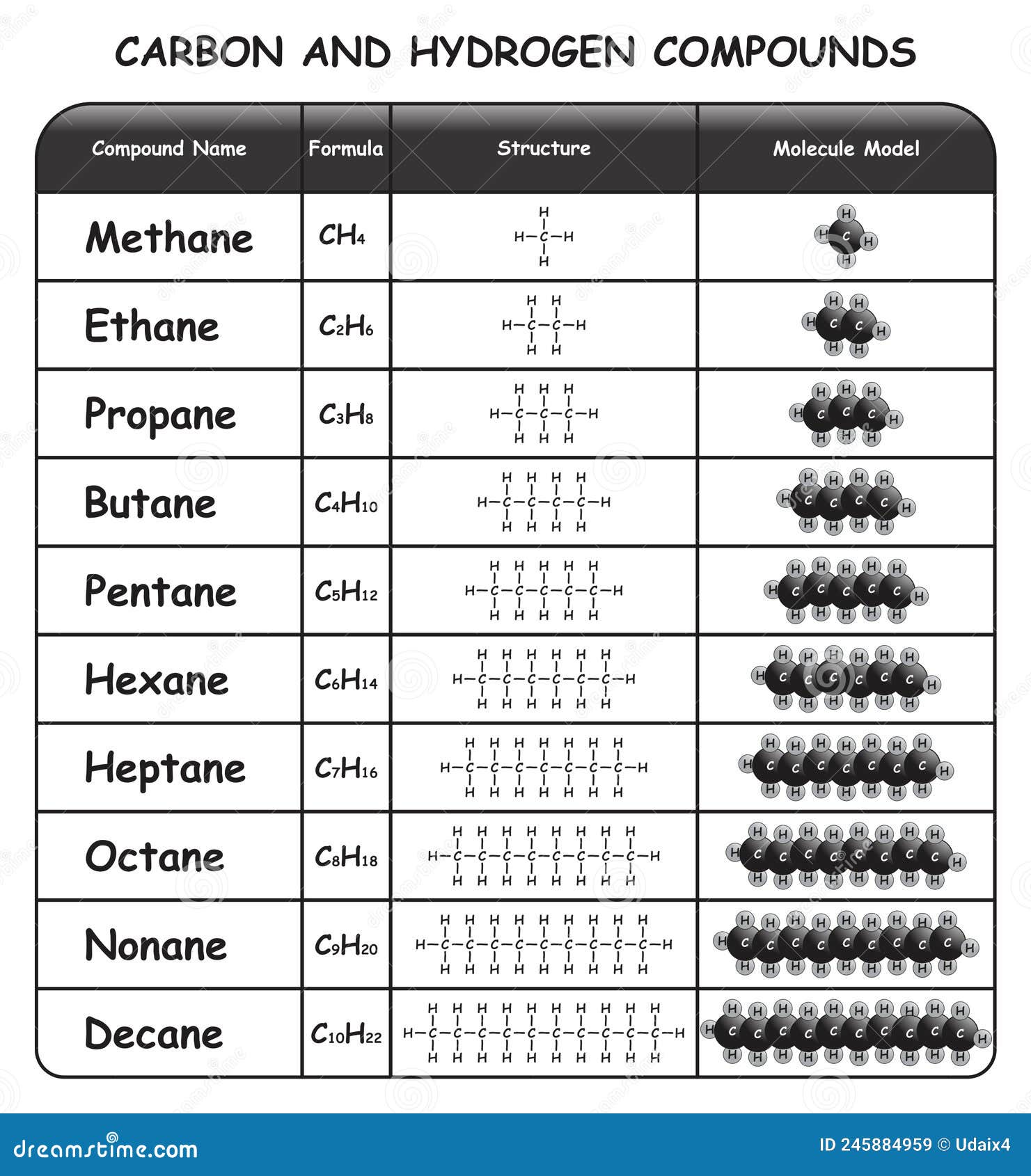
Carbon and Hydrogen Compounds Infographic Diagram Stock Vector - Illustration of chemistry, lesson: 245884959
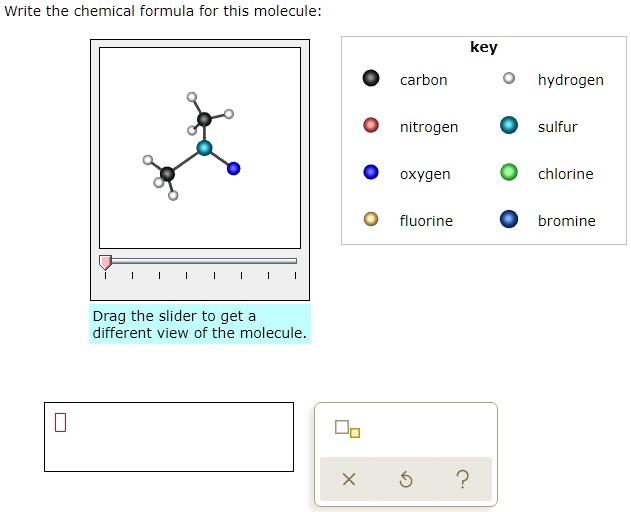
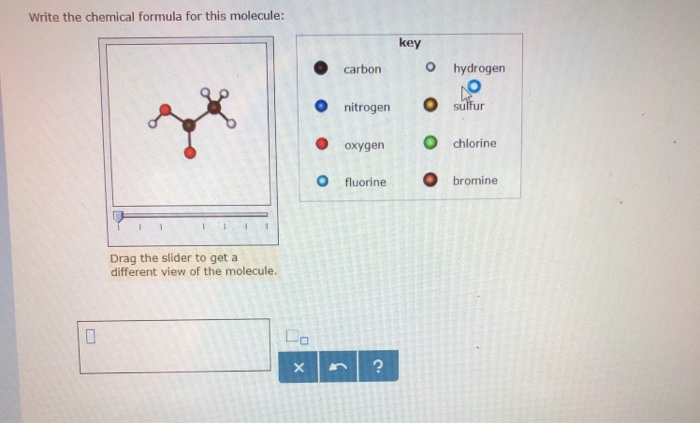
SOLVED: Write the chemica formula for this molecule: key carbon hydrogen nitrogen sulfur oxygen chlorine fluorine bromine Drag the slider to get different view of the molecule

Calculate the Empirical Formula for a compound with the following composition: 46.16% carbon; 53.84% nitrogen 1)Change % to grams (if needed) 2)Convert. - ppt download
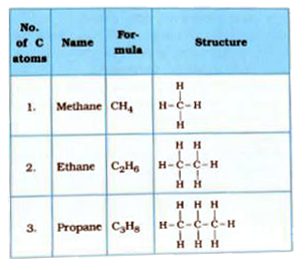
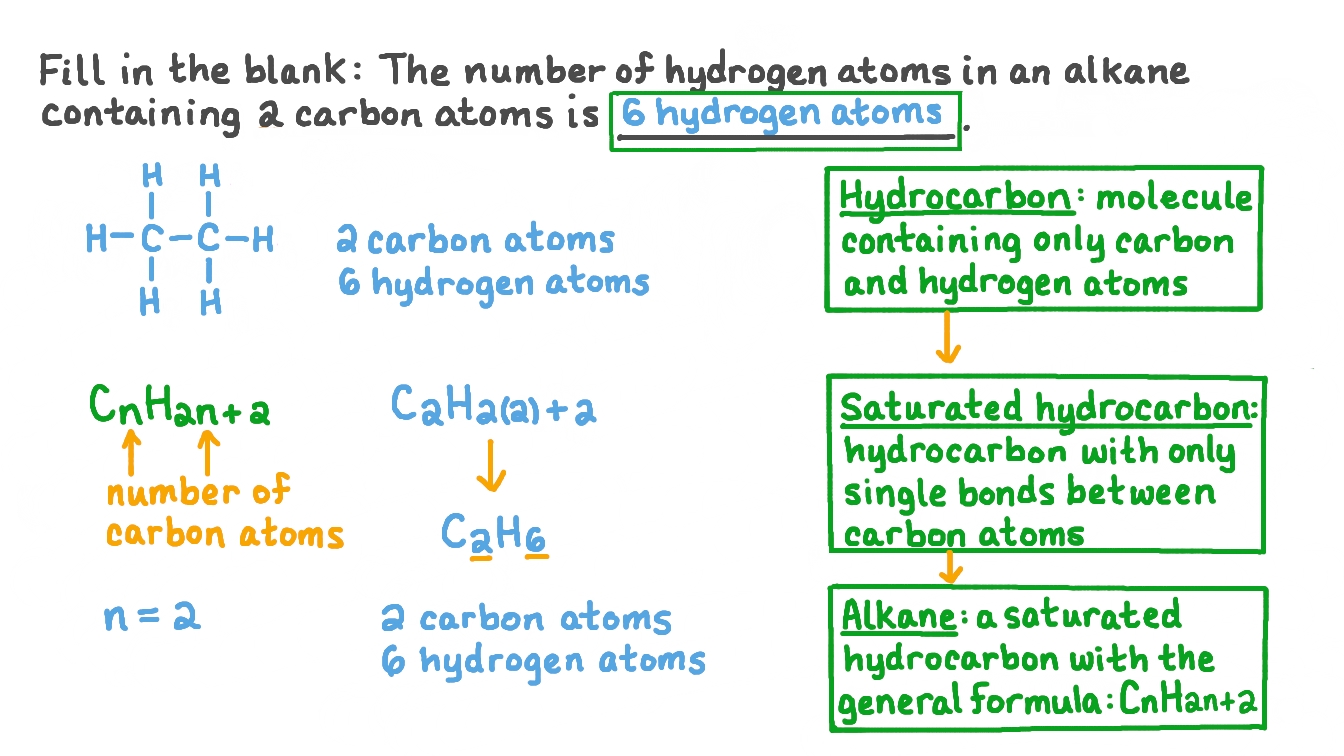
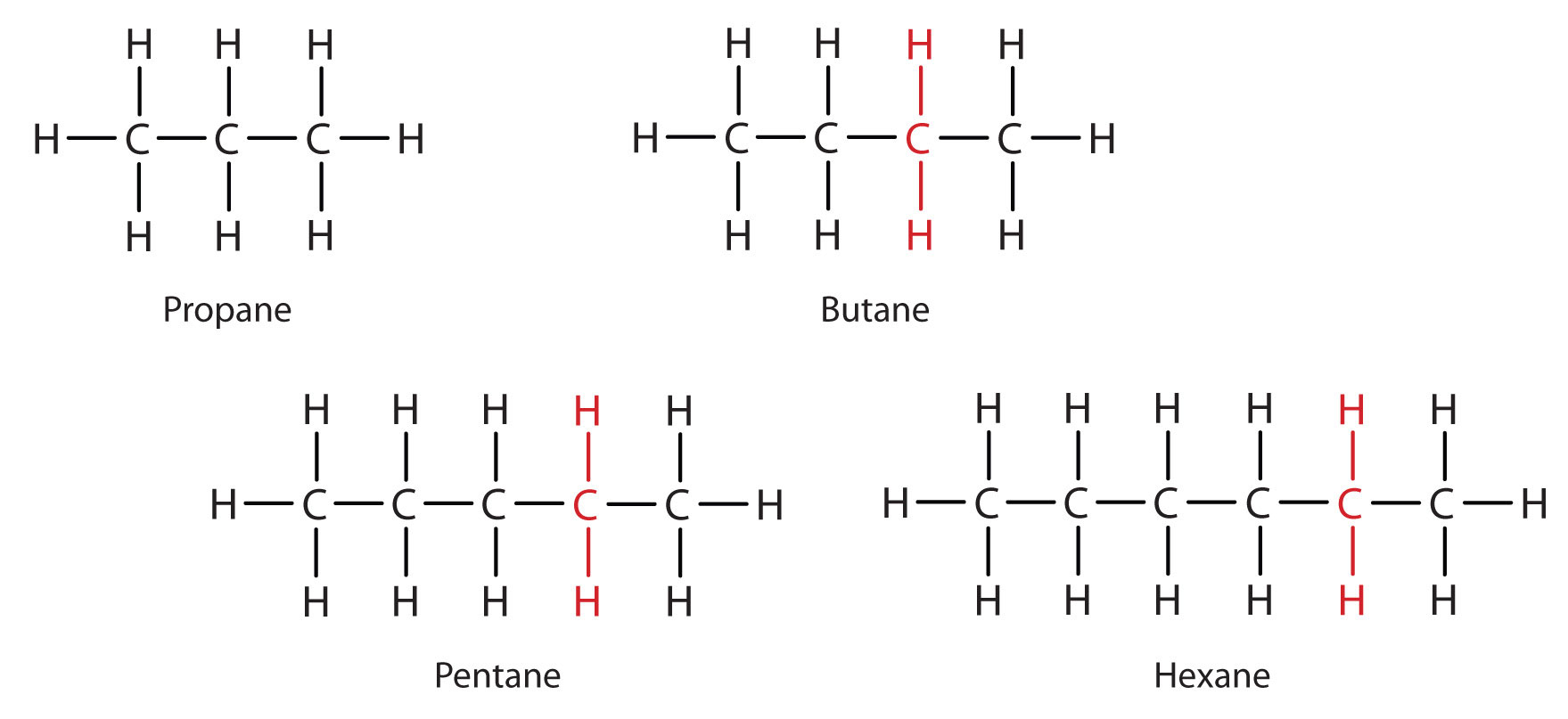
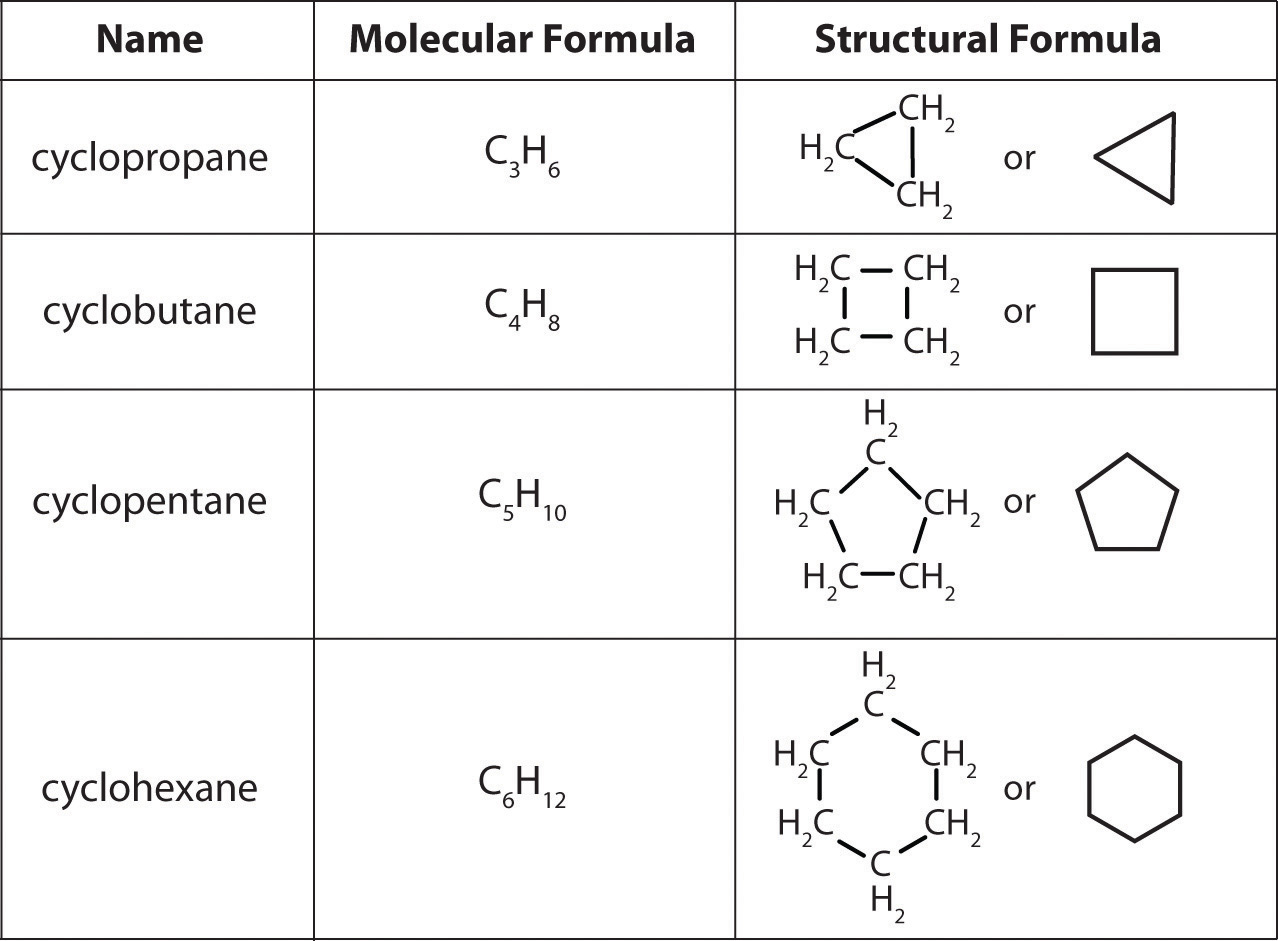
Mention the names, molecular formulae and structures of first six saturated compounds of carbon and hydrogen.
Table of atoms in molecules, chemical formula of carbon,oxygen,hydrogen and nitrogenmolecules.Educational and study content of chemistry and science s Stock Vector Image & Art - Alamy